UK approves world's first gene-editing drug
The UK's Medicines and Healthcare products Regulatory Agency has approved the world's first gene-edited drug to treat sickle cell disease.
The UK's Medicines and Healthcare products Regulatory Agency has approved the world's first gene-edited drug to treat sickle cell disease.
The decision, made on November 17, could save thousands of lives in this country. The drug, called Casgevy , is produced by Vertex Pharmaceuticals . The drug is based on CRISPR gene editing technology, which brought scientists the Nobel Prize in 2020. The subjects who can use the drug are people with sickle cell disease and thalassemia aged 12 and older.
The decision was based on a study of 29 sickle cell patients. After taking the drug, 28 had no serious problems for at least a year. In a study of 42 thalassemia patients, 39 did not need a red blood cell transfusion for at least a year afterward.
Until now, the only long-term treatment for both diseases has been a bone marrow transplant. However, this is an extremely arduous procedure with many unpleasant side effects. According to Dr. Helen O'Neill of University College London, the future of life-changing treatments lies in CRISPR gene editing technology.
Casgevy works by targeting problematic genes in a patient's bone marrow stem cells, which help the body produce normal-functioning hemoglobin.
First, the patient receives chemotherapy. Then, the doctor takes stem cells from their bone marrow and edits the gene in the lab. The cells are then infused back into the patient for long-term treatment. The patient must be hospitalized at least twice. The first time to collect the stem cells, the second time to receive the edited cells.
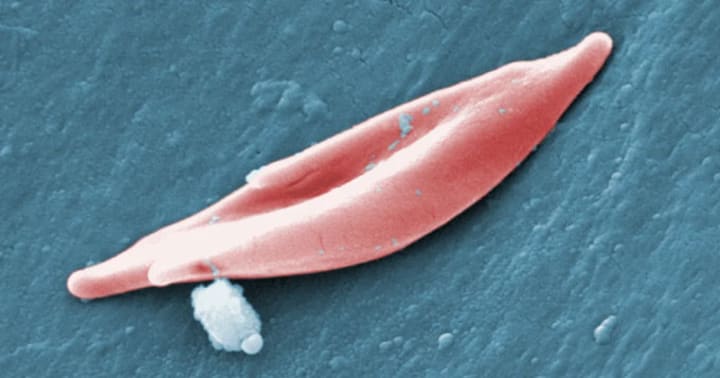
A sickle-shaped red blood cell
Dr. James LaBelle, director of the pediatric stem cell and cellular therapy program at the University of Chicago, said this could be a new wave of treatments for sickle cell patients. The UK approval sets the stage for a similar decision in the US.
The US Food and Drug Administration is also reviewing the drug and another potential treatment, with a decision expected in early December.
Sickle cell disease and thalassemia both result from defects in the gene that carries hemoglobin, the protein in red blood cells that carries oxygen.
In people with sickle cell disease, a genetic mutation causes cells to take on a sickle shape, blocking blood flow and causing severe pain, organ damage, strokes and other problems. The disease is especially common in people of African or Caribbean descent.
For people with thalassemia, the gene mutation causes severe anemia. Patients need blood transfusions every few weeks, injections, and lifelong medication. The disease primarily affects people of South Asian, Southeast Asian, and Middle Eastern descent.
Last year, Britain approved a treatment for a fatal genetic disorder that costs £2.8 million ($3.5 million).
Until now, the only long-term treatment for both diseases has been a bone marrow transplant. However, this is an extremely arduous procedure with many unpleasant side effects. According to Dr. Helen O'Neill of University College London, the future of life-changing treatments lies in CRISPR gene editing technology.
Casgevy works by targeting problematic genes in a patient's bone marrow stem cells, which help the body produce normal-functioning hemoglobin.
First, the patient receives chemotherapy. Then, the doctor takes stem cells from their bone marrow and edits the gene in the lab. The cells are then infused back into the patient for long-term treatment. The patient must be hospitalized at least twice. The first time to collect the stem cells, the second time to receive the edited cells.Dr. James LaBelle, director of the pediatric stem cell and cellular therapy program at the University of Chicago, said this could be a new wave of treatments for sickle cell patients. The UK approval sets the stage for a similar decision in the US.
About the Creator
HK Decor
Telling stories my heart needs to tell <3 life is a journey, not a competition
If you like what you read, feel free to leave a tip,I would love some feedback
https://sites.google.com/view/hk-decor/trang-ch%E1%BB%A7
Enjoyed the story? Support the Creator.
Subscribe for free to receive all their stories in your feed. You could also pledge your support or give them a one-off tip, letting them know you appreciate their work.





Comments
There are no comments for this story
Be the first to respond and start the conversation.